Projects
Molecular regulation of the β-catenin destruction complex
The WNT/β-catenin pathway revolves around the regulation of cysolic levels of the transcriptional co-factor β-catenin. In the absence of WNT proteins, β-catenin levels are kept low by a multiprotein complex known as the β-catenin destruction complex. The scaffold proteins APC and Axin are the main organizers of this complex and mutations in their corresponding genes are causal to cancer development (reviewed in Nat. Rev. Cancer, 2020).
We investigate the molecular mechanisms underlying the regulation of the β-catenin destruction complex in healthy tissue. In addition, we study how cancer mutations disturb the structural organization and function of this complex. Our recent work demonstrated that Axin missense cancer mutations drive ligand-independent pathway activation by an unforeseen mechanism that involves the formation of small-scale Axin aggregates (Nat. Struct. Mol. Biol., 2016). We envision that a deep mechanistic understanding of how cancer mutations disrupt the β-catenin destruction complex will facilitate the development of strategies to modulate WNT pathway activity in cancer (reviewed in Br. J. Pharmacol., 2017).
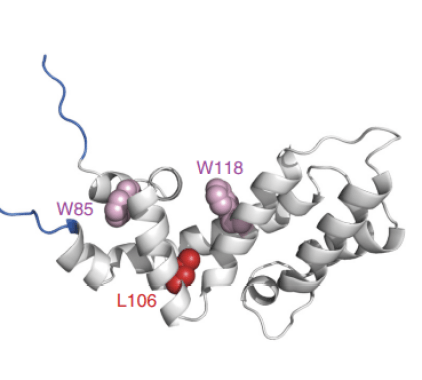
Ribbon structure of the Axin RGS domain. Mutations (in red) that hit the hydrophobic core of this domain drive tumorigenesis (Anvarian et al. 2016)

Biomolecular condensates formed by overexpressed Axin-GFP
Over recent years, multiple groups demonstrated that the β-catenin destruction complex forms liquid-liquid phase separated structures. We examine the role of the different scaffold proteins of the WNT pathway in the assembly of these biomolecular condensates. In addition, we study how mutations in components of the WNT pathway alter these macromolecular structures and the underlying dynamic protein interactions to drive aberrant pathway activation.
Molecular regulation of the WNT receptor complex in homeostasis and cancer
WNT binding to cell surface receptors is decisive for downstream gene activation but the regulatory mechanisms that operate at the receptor level remain poorly understood. We aim to uncover how cells interpret WNT signals received at their cell surface and how dysregulation of receptor-mediated signal relay by mutations leads to cancer.
Our lab elucidated the molecular basis of the first known step in WNT/β-catenin signaling, the interaction between the Frizzled (FZD) receptor and its cytoplasmic effector Dishevelled (Dvl). Using peptide libraries, biochemistry and imaging techniques, we shed new light on how FZD and Dvl communicate to drive downstream β-catenin-mediated transcription (Proc. Natl. Acad. Sci., 2012).

Model for binding of the Dishevelled DEP domain to a discontinuous motif in the WNT receptor Frizzled

The membrane-bound E3 ligase RNF43 mediates removal of the WNT receptor Frizzled (FZD) from the plasma membrane
In the same year, we reported on the discovery of stem cell-specific E3 ligase RNF43 as a novel tumor suppressor that downregulates WNT receptors to inhibit tumor growth (Nature, 2012). Moreover, our findings implicated that mutation-induced loss of RNF43 generates a state of WNT-dependent growth and sensitizes cancer cells to treatment with anti-WNT-based therapy. We currently investigate how unconventional subsets of RNF43 mutations deregulate the WNT pathway to drive cancer growth, to ultimately improve patient selection for tailored treatment strategies (EMBO J., 2020).
Lastly, we generated various functionally active, internally tagged WNT proteins. This approach allowed us to visualize for the first time endogenous WNT proteins at high resolution in the mouse intestine, and isolate and identify novel regulators of the endogenous WNT receptor complex using proteomics (Nature, 2016; Proc. Natl. Acad. Sci., 2018). We are currently generating fluorescent reporters for other less well characterized WNT ligands, to study their function in complex tissues at the endogenous level.
Disease modeling using organoids
Over the past decades, the increased fundamental understanding of the signaling pathways that are essential for tissue renewal has led to the establishment of culturing conditions for adult stem cells in vitro. By using a mixture of growth factors and extracellular matrix components, isolated gut stem cells can be grown into differentiated ‘mini-guts’ or organoids. Nowadays, organoid culturing conditions are established for many tissues, including intestine, pancreas and liver. Moreover, organoids can be grown from tumor biopsies, allowing us to study patient-derived tumors ex vivo.
Given their strict dependence on WNT signaling, intestinal organoids offer an unprecedented opportunity to examine WNT pathway regulation in normal physiology and cancer (Trends Cell Biol., 2020). By altering the dosage of WNTs and other growth factors in the culture medium, intestinal organoids can be driven towards full stemness or (terminal) differentiation. We make use of these properties to study the impact of cancer mutations on WNT signaling and to evaluate novel strategies to target WNT-sensitive cancers (Nature, 2012; Nat. Comm., 2019).

Small intestinal organoid showing WNT-producing Paneth cells (in green) at the bottom of the intestinal crypts. Cortical actin (red) and nuclei (blue) are shown as well.
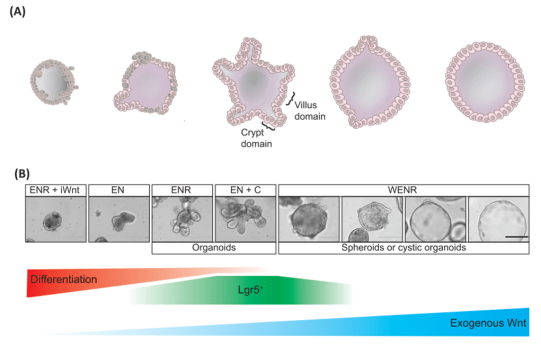
Schematic representation (A) and brightfield images (B) of the effects of increasing dosage of WNT signaling on small intestinal organoid viability and morphology
The emerging possibilities of gene-editing offered by CRISPR-Cas9 technology enable us to introduce genetic alterations in organoids and study their effects on cell behavior in complex tissue. We make use of this approach in various projects in the lab.
The tumor suppressor gene LKB1 is mutated in Peutz-Jeghers Syndrome. Patients develop many hamartomatous polyps in the intestine, and have an increased risk of cancer development. By using genetically engineered small intestinal organoids, we study the effects of LKB1 mutations on tissue organisation and cellular signaling.
Colorectal cancer (CRC) is driven by mutations in multiple signaling pathways, including WNT/β-catenin and EGF/MAPK signaling. CRC subtypes often present with a defined combination of WNT and EGF pathway mutations. We make use of gene-editing in patient-derived CRC organoids of different subtypes to elucidate how the combined action of co-occurring mutations in the WNT and EGF pathway drives cancer development and progression.
Targeting signaling pathways in stem cells and cancer
We employ our fundamental knowledge on signaling pathways and mutations to develop novel therapeutic strategies.
Although RNF43/ZNRF3-mutant cancers are sensitive to WNT blocking agents (Nature, 2012), such reagents may cause a significant level of toxicity. As a more selective approach, we developed nanobodies that block WNT3 binding to the co-receptor LRP6. These reagents inhibit growth of RNF43/ZNRF3-mutant intestinal tumor cells by driving terminal differentiation (Nat. Comm., 2019).
In ongoing work, we investigate the mode of action of small compounds that inhibit the growth of selective cancer subsets, and we develop innovative strategies for targeted protein degradation. Lastly, in collaboration with Prof. Enrico Mastrobattista, we employ protein-decorated liposomes, versatile biocompatible membrane vesicles, for targeting and selective drug delivery to stem-like cancer cells (link).

Protein-decorated liposomes (green) are taken up by selective cell types in the intestinal crypt. Nuclei (blue) and F-actin (red) are also stianed.



